Neat Info About How To Prevent Corrosion Of Iron

1)many different types of coatings can be applied to the surface of the exposed metal in order to prevent corrosion.
How to prevent corrosion of iron. Coatings, when selected and applied carefully, work. A galvanized surface protecting iron alloys with a coating of a more active metal through the process of galvanizing prevents the alloys from corroding. Some popular methods of iron corrosion prevention include:
How can we prevent iron corrosion? Another prevention of corrosion method is using corrosion inhibitors. How can we prevent corrosion of iron?
An understanding of the activity series investigated in experiment 6 suggests that one way of preventing the corrosion of iron is to protect it with a more active metal. The biggest problem with carbon steel is that it contains a lot of iron. Following are the methods which are usually adopted to prevent the corrosion of ferrous metals:
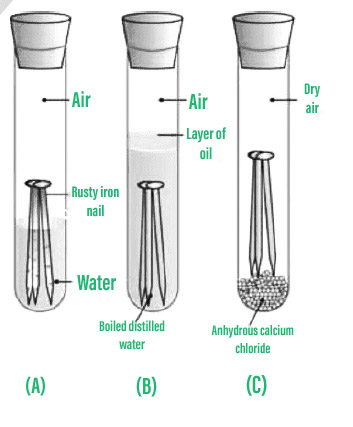
Preventing corrosion removing substances that cause rusting rusting can be prevented by keeping oxygen or water away from the iron or steel: We would like to show you a description here but the site won’t allow us. Oxidization (a chemical reaction between iron and oxygen) results in rusting on the surface of the metal.
What are three ways to prevent corrosion? This can be done by coating the metal being used with paint or enamel. Embedding in cement concrete 4.
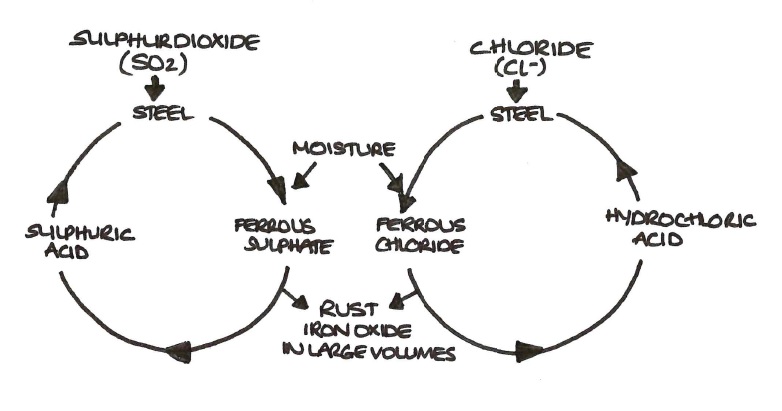
This coating of what is called a “sacrificial metal”. But oxidization is just one example of corrosion. Savings through sound corrosion management •improve education and training of staff in the recognition of corrosion control •implement advanced design practices for better corrosion.