Here’s A Quick Way To Solve A Info About How To Find Out The Atomic Mass

Learn the steps to calculate the atomic mass from.
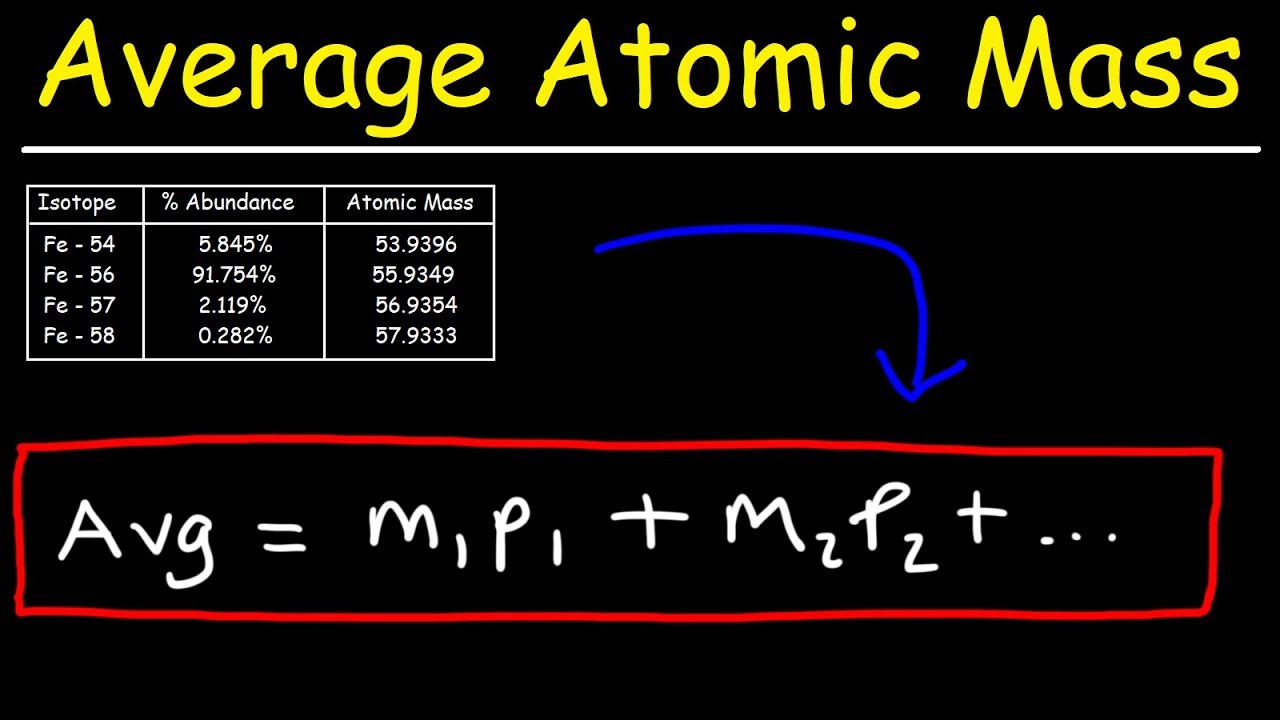
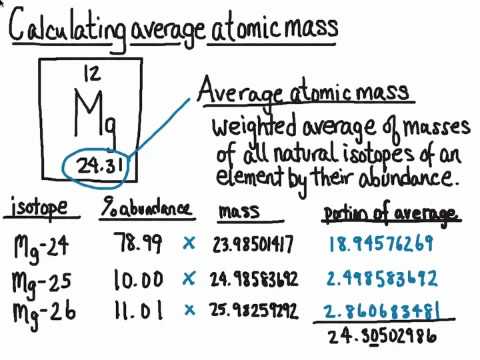
How to find out the atomic mass. For each isotope, multiply its mass by the percent. The relative atomic mass or average mass can be computed from the compositions percentage easily percent. \ [\textit {a}_\textup {r}=\frac {total~mass~of~atoms} {total~number~of~atoms}=\frac { (35\times75)+ (37\times25)} {.
Number of electrons = atomic number; To work out the relative atomic mass of an element all you. How to calculate the atomic mass of an atom, a specific isotope’s atomic mass corresponds to its total mass expressed in dalton (u), also called unified atomic mass units.
To calculate the relative atomic mass, ar, of chlorine: The average atomic mass of various elements are determined by multiplying the atomic mass of each isotope by its fractional abundance and adding the value obtained. In other words, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.
To calculate the relative atomic mass, ar, of chlorine: Identify the percentage of each isotope in the composition of the element and its mass. The atomic number of a sodium atom is 11 and its mass number is 23.
The atomic mass calculator provided here will help you to know how to find the atomic mass calculator of an atom using the formulas. But, since the abundance is in %, you must also divide each. The mass numbers of its isotopes, the abundance of these isotopes, the formula that can be used to calculate the relative atomic mass:
This chemistry video tutorial explains how to calculate the average atomic mass of an element given the percent abundance of each isotope.my website:










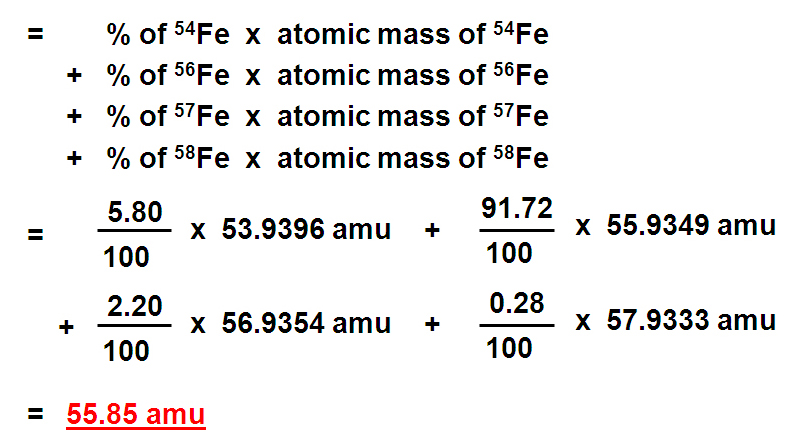

/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)

